In July 2015, a train carrying Bakken crude oil derailed in Culbertson, Montana resulting in an oil spill of 35,000 gallons — more than the contents of a full rail tank car.
But unlike all of the other Bakken train accidents where large amounts of oil were spilled something odd happened. There was no explosion or fire.
So what was different about the accident in Culbertson, Montana?
One potential explanation was that the oil was significantly less volatile than the oil involved in other Bakken accidents that resulted in fires and explosions. The Federal Railroad Administration told DeSmog that two samples of the oil were taken and the Reid vapor pressure for those samples were 8.73 psi and 9.23 psi.
Reid vapor pressure is used to quantify the volatility of substances like crude oil and gasoline. If there are more natural gas liquids in the crude mixture — like propane and butane — it will have a higher Reid vapor pressure (RVP).
In comparison, the oil involved in the massive fire and explosion in Mount Carbon, West Virginia had an RVP of 13.9 psi according to the Wall Street Journal. Samples taken from the train derailment and fire in Lynchburg, Virginia averaged RVP values over 14 psi.
While the initial oil-by-rail regulations released in 2015 refused to address the issue of vapor pressure and volatility, the Pipeline and Hazardous Materials Safety Administration (PHMSA) announced earlier this month that it was “considering revising the Hazardous Materials Regulations (HMR) to establish vapor pressure limits for unrefined petroleum-based products.”
According to the announcement “PHMSA is currently assessing the merits of a petition for rulemaking submitted by the Attorney General of the State of New York regarding vapor pressure standards for the transportation of crude oil. The petition requests that PHMSA implement a Reid Vapor Pressure (RVP) limit less than 9.0 pounds per square inch (psi) for crude oil transported by rail.”
9.0 psi is the industry standard for stabilized oil that is moved in pipelines or on ocean-going tankers and in the petition from the New York Attorney General it notes that pipeline operators in Texas have the right to reject oil with a vapor pressure higher than 9.0 psi.
The high vapor pressure and volatility of the Bakken oil moved by rail has clearly created a concern for regulators ever since the disaster at Lac-Megantic. Secretary Foxx had asked that the original oil-by-rail regulations include volatility limits but was informed by the White House that this would not happen in the rule.
American Petroleum Institute – “a very dangerous conversation”
In October, at the annual Energy by Rail Conference in Arlington, Virginia, Suzanne Lemieux of the American Petroleum Institute (API) gave a presentation on crude oil volatility and stabilization with the title of “Crude Oil Volatility: Myth vs. Fact” — and she was pretty clear about what she thought was a myth.
“I will reiterate that volatility in itself is a myth as far as a one-size-fits-all solution to this problem,” Lemieux said, “It’s not the way to go as far as improving safety.”
Lemieux went on to make a statement about Bakken oil that ignores all of the known science.
“I would say that all of these conversations about [how] Bakken is inherently more dangerous, it’s more volatile, etcetera, etcetera, those things from a chemical properties perspective just aren’t true.”
And while these are controversial statements as they directly contradict the known science, Lemieux made it clear that the API thinks the real danger is even just talking about this topic.
“And so we in the oil and gas industry see this as a very dangerous conversation. Because the main point is that you’re not really reducing risk.”
Perhaps Lemieux and the API were aware that PHMSA was planning to announce this rulemaking as she also spoke against just such a potential outcome.
“And if we were to arbitrarily mandate a national volatility standard as some people have suggested it would really cause a lot of problems and add some additional risk into the supply chain itself.”
Lemieux stressed that this idea was a “huge concern” for the API.
“So it is a huge concern for us that there are these conversations that talk about volatility in these kind of simplified terms because there is no simple answer and it’s not a simple issue.”
While none of this is surprising — it echoes what the API and other industry lobbyists have been saying ever since Lac-Megantic — it does contradict the known science and indicates that the API will fight any new regulations requiring stabilization of oil to lower volatility.
Department of Energy Still “studying” Issue
Lemieux repeatedly cited the work of the Department of Energy (DOE) and other government agencies (including PHMSA) as proof that volatility was not a safety factor worth considering when it came to transporting oil by rail and stabilization of oil to reduce volatility would not decrease risk:
“So we know from several studies and several government agencies including the Department of Transportation, PHMSA within DOT, the Department of Energy and NTSB, that volatility is not the right measure of what makes crude oil safe or unsafe.”
Yet a PHMSA study on Bakken crude reached the following conclusion, contradicting her statement:
“Operation Classification has determined that the current classification applied to Bakken crude is accurate under the current classification system, but that the crude has a higher gas content, higher vapor pressure, lower flash point and boiling point and thus a higher degree of volatility than most other crudes in the U.S., which correlates to increased ignitability and flammability.”
Additionally, the DOE study Lemieux cited makes it very clear that stabilization reduces the volatility of oil.
“The principal purpose of stabilization, in contrast to conditioning, is to remove hydrocarbon compounds—which possess fuel value, but which also possess higher vapor pressures (i.e., have lower boiling points)—so as to reduce the volatility of the crude oil.”
That DOE study also concluded, “it is likely that a combination of crude oil properties—especially those associated with potential for flammable vapor formation—could be used to predict combustibility.”
In a September 2014 hearing on the risks of Bakken oil, Christopher Smith, Assistant Secretary for Fossil Energy at the DOE stated the following: “We think that in a laboratory setting for crude oil – higher volatility is going to be consistent with higher light ends – which do have a higher degree of flammability and volatility.”
So it is clear the DOE understands that having more light ends (aka natural gas liquids) in the crude oil increases the volatility which correlates to a higher degree of flammability and volatility. And PHMSA has reached the same conclusion. The DOE-sponsored report also states that stabilizing oil reduces these light ends.
Despite this, the DOE has contracted Sandia National Laboratories to further study the issue of crude oil volatility and flammability. Lemieux said the study was expected to be completed sometime in 2018 because they were still just collecting samples of the oil. Clearly there is no sense of urgency to complete this study.
Meanwhile some people believe that the study in itself is pointless. In April of 2015, industry publication Railway Age asked the DOE why they needed to do further research on what is known and established science. In reporting on the lack of response from DOE, Railway Age was quite blunt in assessing the situation:
“There was no response from the Department of Energy to our request for more information about the study, specifically why it needs two more years to figure out what by now should be obvious to the dullest high school chemistry student.”
In her opening remarks to the Energy by Rail conference, Susan Lemieux made it quite clear that she is not a scientist saying, “Just to preface my presentation I am not a petroleum engineer and nor am I a chemical engineer…” She followed this with the statement “what we’re trying to get to is to understand what crude oil is.”
While completely unbelievable, the industry’s position — despite spending billions a year on research — is that it doesn’t understand crude oil.
But, to be fair, perhaps that is what happens when you don’t ask scientists. Lemieux repeatedly stated how complex this was and that only the scientists really understood it.
In April 2015, Al Jazeera asked an actual oil scientist, Ramanan Krishnamoorti of the University of Houston, whether more study was required to understand the volatility of crude oil and he also was quite blunt.
“The notion that this requires significant research and development is a bunch of BS. The science behind this has been revealed over 80 years ago, and developing a simple spreadsheet to calculate risk based on composition and vapor pressure is trivial. This can be done today.”
And there you have it. When you ask a scientist, that is the answer you get. The science is known and the risks could be quantified today.
Yet the DOE is now in the process of studying the issue for years and the API claims they still don’t understand the science and that even talking about this issue is a “very dangerous conversation.”
All of which delays any regulations that would improve safety and protect workers and those living near the tracks.
Crude Oil Doesn’t Explode Like That
After the disaster at Lac-Megantic, Matthew Goitia, chief executive of Peaker Energy Group LLC, a Houston based crude-by-rail company told the Wall Street Journal that, “Crude oil doesn’t explode like that.”
Which is true. Traditional crude oils with low amounts of natural gas liquids and much lower vapor pressure and volatility do not explode like that. Which is why if you stabilize light volatile oils like the Bakken crude and remove the natural gas liquids you will get a crude that “doesn’t explode like that.”
So, the real question is, what should that vapor pressure be to make oil-by-rail safe? Is 9 psi low enough? While the lack of fire or explosion in the Culbertson derailment and spill certainly indicates the lower RVP in that incident may have prevented the oil from igniting, would an even lower RVP be safer?
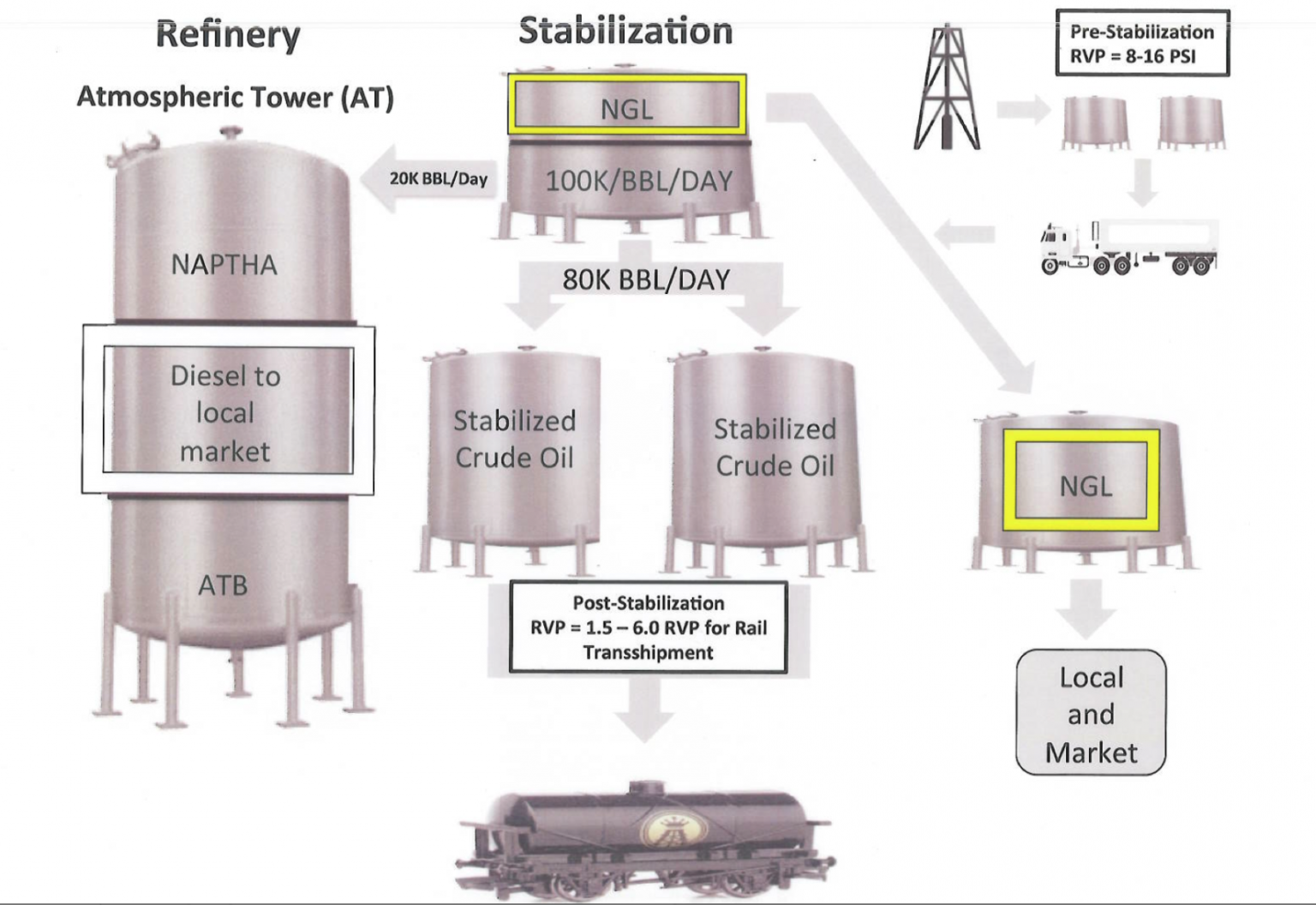
As reported by DeSmog, prior to the new oil-by-rail regulations, stabilization company Quantum Energy met with federal regulators and presented information that explained how regular crude oil has an RVP of 5-7 psi and Bakken crude has an RVP between 8-16 psi.
The last slide in the Quantum presentation shows that “post stabilization” Bakken crude would have a RVP of 1.5 – 6 psi.
If regular crude, the crude oil that “doesn’t explode” has a vapor pressure of 5-7 psi, it would seem like that would be a good starting point — if safety is the goal.
For more information on the history on the efforts to address the volatility of Bakken oil, see this video produced by DeSmog.
Main Blog Image: Slide from Quantum Energy presentation to OIRA.
Subscribe to our newsletter
Stay up to date with DeSmog news and alerts